Projects
- ISAS Small Science Program
- JSPS Scientific Research(S)
- NEW: JSPS Scientific Research(S)
ISAS Small Science Program: DUST (Determining Unknown yet Significant Traits)
Working Group: DUST Nucleation
Term of Project: FY 2017-2024
PI: Yuki Kimura (Hokkaido University)
Great objective
Elucidate a whole processes of material evolution from molecules, dust, planet and to organics in a history of the universe based on fundamental physical and chemical properties of dust.
Why cosmic dust?

“Most of the atoms in the interstellar medium exist in the gas phase. Only about 1% of the total mass of the elements forms tiny solid particles called grains. Despite their low abundance, these grains are significant as building blocks of planetary systems, as substrates for the formation of molecules, as energy transducers in interstellar and circumstellar environments, and as key players in the efficient formation of stars. The efficiency of these various contributions depends strongly on the chemical composition, size, crystal structure, and geometry of the grains that are initially formed in gaseous outflows of evolved stars and subsequently processed in interstellar environments. Therefore, to understand these characteristics of grains, it is necessary first to clarify the compositions and quantities of grains formed in stellar gas outflows.” (Reference: Y. Kimura,* K. K. Tanaka, T. Nozawa, S. Takeuchi, Y. Inatomi, Pure iron grains are rare in the universe, Science Advances, 3 (2017) e1601992)
Scientific goal of this project is determined most important physical properties (surface tension and sticking probabilities) and infrared spectra during formation and agglomeration of cosmic dust under microgravity condition.
Elucidation of formation processes of silicate dust
Silicates has been distributed ubiquitously in the universe and also main minerals on the Earth and, therefore, understanding its formation process are very much important. We will determine two crucial physical parameters, sticking probability of molecules and surface free energy by means of interferometry (Figures 1-3), and obtain infrared spectra (Figure 4) during formation of silicate dust by microgravity experiments.

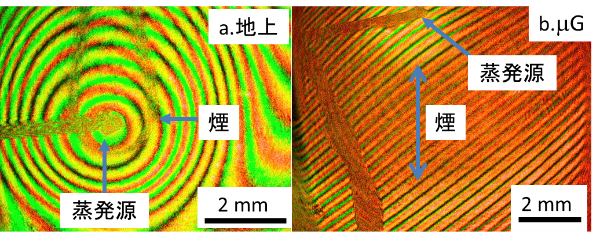
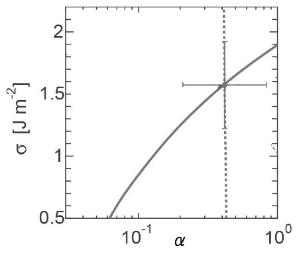
Elucidation of formation processes of carbonaceous dust
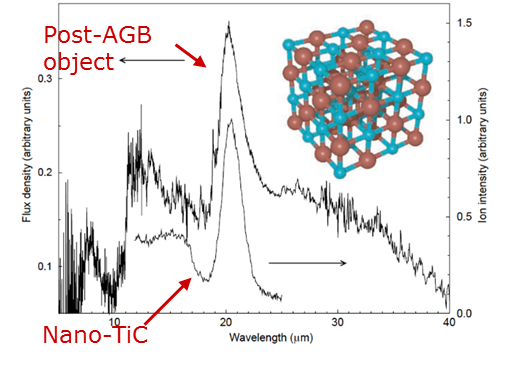
Determine the physical parameters of carbon and titanium carbide (TiC) dust and elucidate formation condition of core-mantle dust (Figure 5) is an objective of this project. It is still difficult to explain the thickness of the graphitic mantle layer based on the typical physical conditions expected in the circumstellar envelopes of carbon stars (Reference: Lodders & Fegley 1995; Sharp & Wasserburg 1995; Chigai et al. 2002); T. Chigai, T. Yamamoto, C. Kaito & Y. Kimura, ApJ, 587 (2003) 771.). Infrared spectrum of TiC makes a strong constrain on the origin of 21 mm feature of C-rich star (Figure 6).

Why microgravity experiments using overseas rockets?
We are planning to use sounding rockets in US and Sweden together with Dr. Joseph A. Nuth III of NASA Goddard Space Flight Center and Prof. Jürgen Blum of Institute for geophysics and extraterrestrial physics. Following is the reason why we need international collaborations.
Why microgravity experiment is required? The reasons are Homogeneity of nucleation environment, slower cooling rate, monodispersivity of products and longer duration for infrared spectroscopy.
Why we use sounding rockets? The reasons is to wait suppression of turbulence of buffer Ar gas and homogenize the temperature of an evaporation source. In addition, several tens sec are required to measure the signal changes of infrared spectra due to crystallization. To suppress a convection current generated by an evaporation source, gravity condition less than 0.01% (10-4 G) is necessary.
Why overseas rocket is necessary? Recovery of the sample allows us to determine size, number density, crystalline structure and crystallinity.
JSPS, Grant-in-Aid for Scientific Research(S):Nucleation
Term of Project:FY2015-2019
PI: Yuki Kimura (Hokkaido University)
Objective
The early stages of crystallization, called “nucleation” is an essential process because of beginning of material formation and importance in various fields. I believe significant properties of nano-materials and hydrated layer on a material play a critical rule. In this project, we tackle nucleation by in-situ observation using originally developed transmission electron microscopy and multi wavelength laser interferometry. Physical and chemical mechanisms in the nucleation processes will be elucidated including a behavior of precursors.
Purpose and Background of the Research
Since “nucleation” is a fundamental process to form a material from atoms or molecules and has been believed to be determined characters of the products, understanding of the mechanism of nucleation is essential for material science. However, the detail of the process is still unknown since the modelling by Gibbs (1876). Recent years, new nucleation models have been proposed and actively debated.
In this project, we will perform in-situ transmission electron microscope (TEM) observation using a liquid cell and show the direct evidence of a relationship between nucleation and precursor. We believe that the keys to understand nucleation are characteristic properties of nanoparticles and hydrated layer. Therefore, physical properties (surface free energy and sticking probability) of nanoparticles will be determined by nucleation experiment from a vapor phase and the results of nucleation in water solution and in ionic liquid solution will be compared to know how hydrated layer works during nucleation. As the result, a new model of nucleation will be constructed.
Research Methods
Recently, we succeeded to make a system to observe a phenomena and a reaction of nanoparticles in a water solution. This system is a most powerful for visualization of nucleation process, which will be progressed in meso-scale. Individual nanoparticles can be observed and analyzed its crystal structure by electron diffraction. In vapor phase experiments, physical properties of nanoparticles will be determined based on supersaturation at the nucleation, which determined by double wavelength Mach-Zehnder-type interferometer (Fig. 1). The results will be compared with that obtained by molecular dynamics simulation or global reaction route mapping. As the result, we will construct an advanced nucleation model.
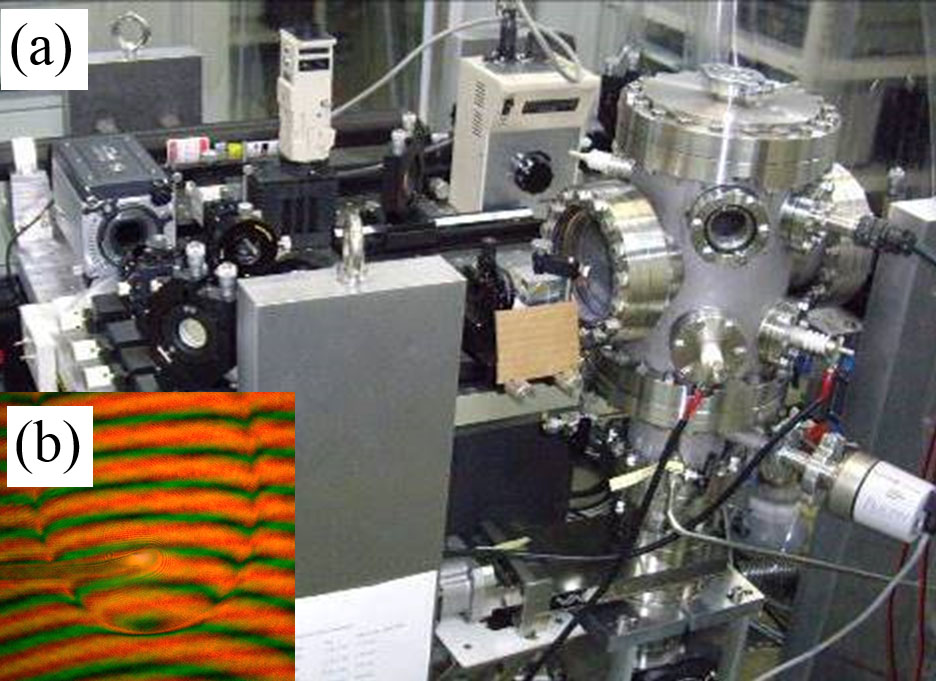
Expected Research Achievements and Scientific Significance
Current view of nucleation based on microscale phenomena by optical microscope will be nanoscale view by TEM. Our results will be contribute to the field of biomineralization and formation of biomaterial in near future.
JSPS, Grant-in-Aid for Scientific Research(S):Paradigm shift in the method for observing non-equilibrium processes in real space: Elucidation of nucleation processes from solution by TEM
Term of Project:FY2020-2024
PI: Yuki Kimura (Hokkaido University)
Objective
In order to understand nucleation, it is necessary to extend the method of structure determination by microscopic observation in equilibrium state, which was established in the 20th century, to dynamic observation in real space of non-equilibrium processes. The purpose of this study is to find the key factors that determine the nucleation route. Then, we aim to construct a nucleation model by elucidating the contribution and material dependence of each factor governing nucleation in solution.
Purpose and Background of the Research
Nucleation is a process whereby particles are formed by the agglomeration of atoms or molecules. Because the nucleation process determines the size, crystal structure, number density, and other properties of the resulting particles, an understanding of its mechanism is crucial for the development of materials science. Nevertheless, our understanding of the physical and chemical processes involved in nucleation remains poor. In this project, our objective is not only to achieve an understanding of nucleation process of individual materials, but also to develop a method for determining the physical properties of nanoparticles and elucidating the roles of dehydration, viscosity, and dimer formation in the nucleation process. Consequently, our final objective is to identify the key factors that determine the nucleation route.
Research Methods
Our main method is the in-situ transmission electron microscope (TEM) observation of nucleation processes from aqueous solutions. In order to understand the role of the hydrated layer, we have also conducted nucleation experiments from ionic liquid solutions and the gas phase. To observe nucleation from solution samples, we use three techniques (a liquid cell holder (Fig. 1), a solution cell, and a graphene film). These techniques allow us to directly observe the growth rate, shape, assembly, arrangement, and size of the resulting crystals and to identify the phases by electron diffraction patterns. Here, we establish a novel dynamic observation method for non-equilibrium processes using machine learning to visualize the entire nucleation process from solution through precursors to crystal formation by in-situ observation using a TEM.
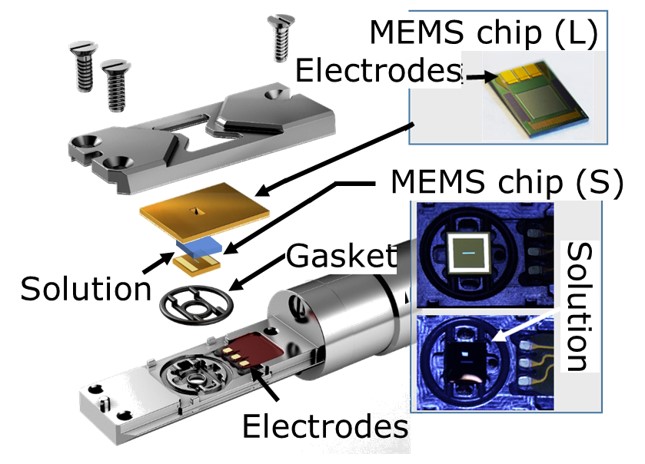
Expected Research Achievements and Scientific Significance
Because our aim is to identify the key factors that govern the nucleation process and then to construct a nucleation model, our project constitutes groundbreaking fundamental scientific research. After our project, we hope to see a new world in which bottom-up processes for producing nanoparticles and crystals from atoms and molecules can be designed. In addition, we hope to understand the processes involved in the formation of cosmic dust, which consists of nanoparticles with a size of less than 100 nm, and which is abundant in the universe in the gas outflow of dying stars. Furthermore, we will identify conditions that are conducive to the precipitation of metastable phases to facilitate the dissolution of medicines. Thus, the significance and impacts of the results are, potentially, extremely wide-ranging.




